Nanobiotechnology
We are a shared laboratory helping other scientists, such as biochemists, chemists and biologists, to better understand the biological processes taking place in living cells, but also to image biomolecules in their natural environment, or complex properties of new nanomaterials. Our labs contribute to a better understanding of heart diseases, the effects of cytostatics on cancer cells or to the development of new treatments of osteoarthritis. We monitor the structure of biomolecules and nanomaterials for the preparation of new types of biosensors. Monitoring cell adhesion then contributes to the development of new implant materials. The main instruments used in our laboratories are AFM microscopes, confocal optical microscope, Raman microscope, biosensor platforms, electrochemical and electrophysiological instruments.
Key Equipment
- JPK NanoWizard® 3 combined with a fluorescence microscope and Olympus FV2100 confocal microscope
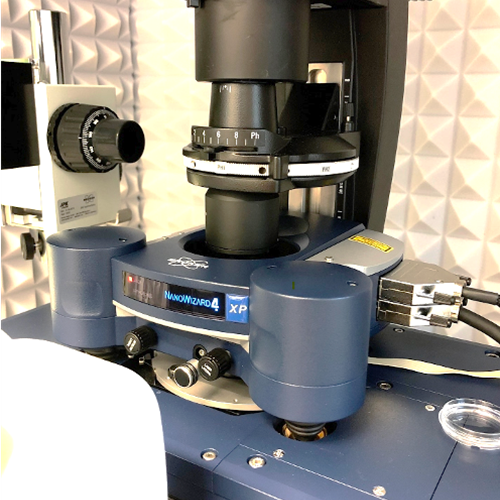
- JPK NanoWizard® 4XP combined with a fluorescence microscope (incl. FluidFM module and Bruker BioSoft NanoIndenter)
- Bruker Dimension FastScan
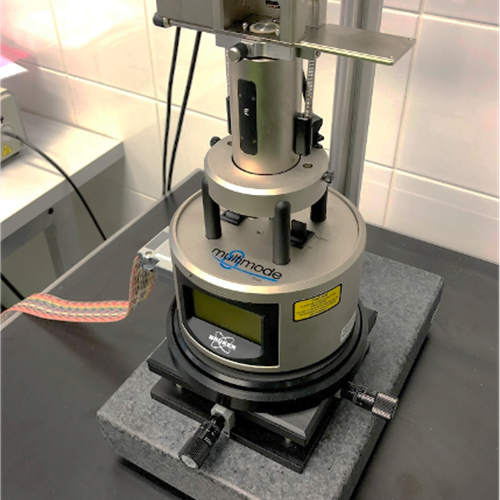
- Bruker Multimode 8-HR
- Entry-level AFM microscope
- Multichannel MEA2100-Mini
- NTMDT NTgra Vita
- Raman microscope Renishaw inVia
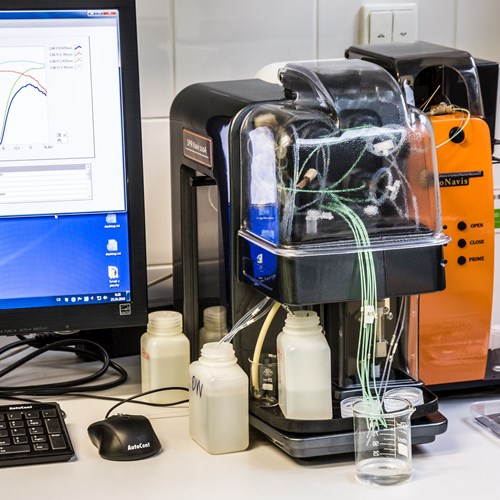
- SPR module with goniometer (SPR Navi 210A)
- Upconversion scanner LABROX
- Electrochemical analyzer (Autolab)
Expertise
- Cells - mechanical properties: living cells in either standard plastic Petri dish (TPP 93040) or special confocal dish (consult us for a list of compatible dishes). Force Mapping of cell cultures, mechanical characterization of cardiomyocytes. Possible combination with optical microscopy (bright field, fluorescence) – independent/overlay imaging with AFM. Standard CO2 incubator and small flow box available in the lab. UV sterilization of the room and workspace every day.
- Cells - imaging: living cells in either standard plastic Petri dish (TPP 93040) or special confocal dish (consult us for a list of compatible dishes). Fixed cells (PFA) on a glass slide. Modes: contact mode, QI, PF-QNM, Force Volume. Possible combination with optical microscopy (bright field, fluorescence) – independent/overlay imaging with AFM. Standard CO2 incubator and small flow box available in the lab. UV sterilization of the room and workspace every day.
- Biomolecules - imaging: AFM imaging of biomolecules (proteins, DNA, macromolecules and biopolymers) and their complexes. Immobilization on mica (muscovite), other materials (HOPG, silicon, metal electrodes) can be used as well. Methods: tapping mode, QI, PF-QNM, Force Volume.
- Nano-objects imaging: AFM imaging of nano-objects (nanoparticles, nanotubes, nanorods, etc.) and their complexes. Immobilization on mica (muscovite), other materials (HOPG, silicon, metal electrodes) can be used as well. Methods: tapping mode, QI, PF-QNM, Force Volume.
- Raman microscopy: Mapping of chemical properties of the samples. Combined with optical and/or AFM imaging.
- Electrochemical measurements: High-end electrochemical analyzer (potentiostat/galvanostat) for voltammetry, amperometry and electrochemical impedance spectroscopy (EIS) on diverse electrodes and surfaces. Possibility of 2-channel measurements, high sensitivity, low noise. SW Autolab Nova for data evaluation.
- Nanodeposition system: Non-contact (ink-jet) deposition of pico- and nano- liter droplets of samples onto diverse substrates. Positioning of the droplets with micrometer precision, creation of various patterns and arrays, protein chip fabrication. Immobilization of proteins and other biomolecules on specific positions in controlled temperature and humidity.
- Fluorescence scanner: Fluorescence scanning of protein, glycan, DNA, cell and other arrays or fluorescently labeled samples. The substrate dimensions are limited to microscope slides. Resolution down to 0.5 um/px. Three fluorescence channels (ex/em): 488/520, 532/572, 635/675 nm. Autosampler for 24 slides. Data evaluation in SW Mapix.
- SPR biosensor: Two-channel SPR (bio)sensor based on surface plasmon resonance. Characterization of optical properties of thin layers in solution and in dry state, and measurement of their changes in real-time. The instrument is a true goniometric SPR providing a wide angular range. Use of 2 laser wavelengths allows to measure the refractive index and layer thickness. Combination with simultaneous electrochemical measurements is possible. Label-free measurement and characterization of biomolecular interactions with one binding partner immobilized on the surface, the other free in solution. Determination of kinetic parameters and binding constants, concentration measurement of various analytes.
- FluidFM: Special combination of AFM with microfluidics, suitable for nanoliter injection/aspiration to/from a single cell; cell adhesion studies, single cell biomechanics/electrochemistry.
- NanoIndenter studies: BioSoft indenter suitable for study of soft biomaterials and biological samples (cells, tissues, hydrogels, etc.)
Open-access
- Preparation of samples for AFM
- Atomic force microscopic (AFM) imaging in contact and non-contact modes in dry and wet conditions, using bare and functionalised scanning tips for bioforce and biointeraction studies
- Combination of AFM with inverted optical microscopy, fluorescence and confocal microscopy, electrochemistry, near-field optical microscopy (SNOM)
- Nanomanipulations, nanolitography, nanopatterning and nanodeposition of biomolecules
Core Facility contact
Jan Přibyl - jan.pribyl@ceitec.muni.cz

Do you want to use this equipment or services?
More information about Nanobiotechnology and related services you can find in document Equipement and services of CIISB .